Groundbreaking Gene-Editing Therapy Receives Green Light, Signaling a New Era in Sickle Cell Treatment
Date: 12 December 2023
Introduction: In a landmark decision, the U.S. Food and Drug Administration (FDA) has granted approval to Vertex Pharmaceuticals and CRISPR Therapeutics for CASGEVY™ (exagamglogene autotemcel), a pioneering gene-editing therapy designed for the treatment of Sickle Cell Disease (SCD). This approval marks a significant breakthrough in the field of genetic medicine, offering hope to individuals grappling with the challenges of this debilitating blood disorder.
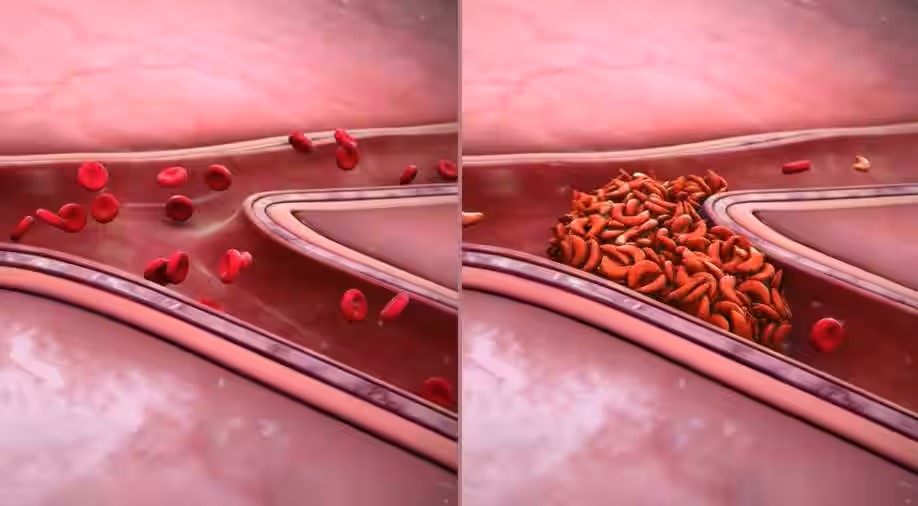
A Transformative Treatment:
CASGEVY™ represents a revolutionary approach to treating SCD, leveraging the power of CRISPR/Cas9 gene-editing technology. The therapy is designed to address the genetic root causes of SCD, potentially providing a curative solution and improving the quality of life for patients.
Here is the comment of Reshma Kewalramani, M.D., Chief Executive Officer and President of Vertex: “CASGEVY’s approval by the FDA is momentous: it is the first CRISPR-based gene-editing therapy to be approved in the U.S. As importantly, CASGEVY is a first-in-class treatment that offers the potential of a one-time transformative therapy for eligible patients with sickle cell disease.” The CEO conveys his gratitude “to the patients and investigators whose trust in this program paved the way for this landmark approval.”
FDA Approval Highlights:
- First-of-Its-Kind Approval: CASGEVY™ is the first CRISPR/Cas9 gene-editing therapy for SCD to receive FDA approval. This signifies a groundbreaking moment in the convergence of advanced genetic science and mainstream medical treatment.
- Precision Medicine Advances: The FDA’s approval of CASGEVY™ underscores the agency’s commitment to advancing precision medicine. By targeting the specific genetic mutations responsible for SCD, this therapy represents a tailored and individualized treatment approach.
- Hope for Patients: For individuals living with SCD, this approval represents a ray of hope. CASGEVY™ holds the potential to transform lives by addressing the underlying genetic factors that contribute to the development and progression of the disease.
CASGEVY™ Mechanism and Benefits:
CASGEVY™ involves the modification of the patient’s own hematopoietic stem cells using CRISPR/Cas9 technology. This targeted genetic editing aims to increase the production of fetal hemoglobin, mitigating the symptoms and complications associated with Sickle Cell Disease.
Benefits for Patients Include:
- Potentially Curative: CASGEVY™ is designed to be a one-time, potentially curative treatment, offering a transformative approach to patient care by addressing the genetic root causes of SCD.
- Improved Quality of Life: Patients with SCD often experience chronic pain, organ damage, and other complications. CASGEVY™ holds the promise of alleviating these symptoms, providing patients with the potential for a healthier and more fulfilling life.
The Path Forward:
CASGEVY received a conditional marketing authorization by MHRA in UK and the National Health Regulatory Authority in Bahrain for patients aged 12 years and older with SCD with recurrent vaso-occlusive crises (VOCs) or transfusion-dependent beta thalassemia (TDT) without a human leukocyte antigen (HLA) matched hematopoietic stem cell donor. The European Medicines Agency and the Saudi Food and Drug Authority are currently reviewing CASGEVY as a treatment for both SCD and TDT.
As for TDT, the use of CASGEVY for this disease in the US remains investigational, even though Vertex has submitted a BLA to FDA for the potential use of this treatment for patients aged 12 years and older with TDT. As for now, the company has been assigned a Prescription Drug User Fee Act (PDUFA) target action dated March 30, 2024.
With FDA approval secured, Vertex Pharmaceuticals and CRISPR Therapeutics are now focused on the next steps, ensuring that CASGEVY™ becomes accessible to patients who stand to benefit from this innovative therapy. The companies are committed to working closely with healthcare providers, regulatory bodies, and the broader medical community to facilitate the integration of CASGEVY™ into the treatment landscape for SCD.