In the ongoing battle against infectious diseases, understanding the intricate mechanisms of bacterial behavior is paramount. Most disease-causing bacteria possess a remarkable ability to rapidly multiply, initiating illness within a matter of minutes. However, equally menacing is their capability to enter a resting state, evading antibiotics and contributing to chronic infections. To shed light on this phenomenon, a recent study, published in the Proceedings of the National Academy of Sciences and titled “Polyphosphate affects cytoplasmic and chromosomal dynamics in nitrogen-starved Pseudomonas aeruginosa,” has delved into the role of polyphosphates (polyP) in regulating bacterial dynamics, particularly during periods of nutrient deprivation.
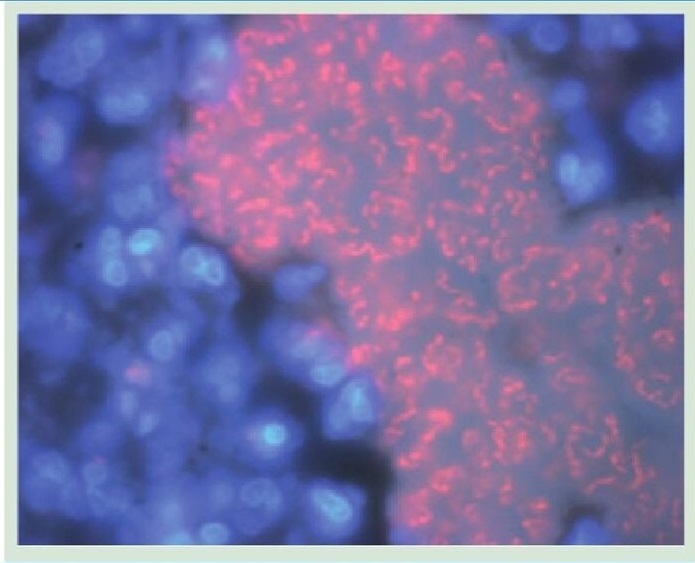
Bacteria in slow-growing and non-growing phase
Lisa Racki, assistant professor in the Department of Integrative Structural and Computational Biology at Scripps Research and senior author of the study, emphasizes the need for novel strategies targeting bacteria’s slow-growing and non-growing phases. She states, “Many current antibiotics block bacterial growth, but bacteria spend a lot of their time not growing. We really need new and creative strategies for targeting bacteria’s slow-growing and non-growing phases.”
The study, conducted by Racki and her collaborators, focuses on Pseudomonas aeruginosa, a notorious pathogen known for causing pneumonia, blood infections, and persistent biofilm-associated infections. One of the reasons why Pseudomonas aeruginosa is hard to treat is because it produces biofilms, which tightly join communities of bacteria, most of which in a resting place, where they are difficult to be reached by antibiotics.
When the bacterium is starved of nitrogen, a key nutrient for bacterial growth, the cell begins to produce polyP. The researchers found out that mutans unable to produce polyP cannot enter the resting phase. Racki describes their findings, stating, “What we found is that when you get rid of polyP, everything in the cell moves too much. The cells are partying when they should be taking a break.”
Role of polyP
In other words, the study reveals that polyP plays a crucial role in facilitating bacterial transition into a resting state. Under nitrogen starvation, P. aeruginosa lacking polyP exhibited heightened cytoplasmic mobility, leading to cellular hyperactivity and impaired ability to enter a quiescent state. This aberrant behavior underscores the significance of polyP in orchestrating bacterial responses to environmental cues.
Furthermore, the researchers observed that polyP influences the partitioning of cellular components, distinguishing between a “more mobile” and a “less mobile” population within the cell. The absence of polyP resulted in increased mobility of small particles within the cytoplasm, indicative of disrupted cellular homeostasis.
Study’s implications for the treatment of bacterial infections
The implications of these findings extend beyond understanding bacterial physiology. Racki emphasizes, “This not only helps point in possible directions for treating pathogenic bacteria, but also reveals answers for fundamental questions about how things diffuse throughout a bacterial cell.”
Looking ahead, Racki and her team aim to unravel the intricate mechanisms underlying polyP-mediated regulation of bacterial physiology. By elucidating the molecular pathways involved, they seek to identify novel targets for combating bacterial infections, particularly those associated with biofilm formation and antibiotic resistance.

Leave a Reply