In a groundbreaking study published in Nature Communications, scientists from the University of California, Los Angeles (UCLA), led by Dr. David Walker, have identified a critical factor in brain aging linked to declining healthspan—the accumulation of F-actin, a filamentous form of actin, in the neurons of aging brains. Their findings, based on research conducted with Drosophila melanogaster (fruit flies), provide compelling evidence that managing actin levels in brain cells can improve neuronal function and slow the effects of aging. This research opens exciting new doors for age-related brain health research.
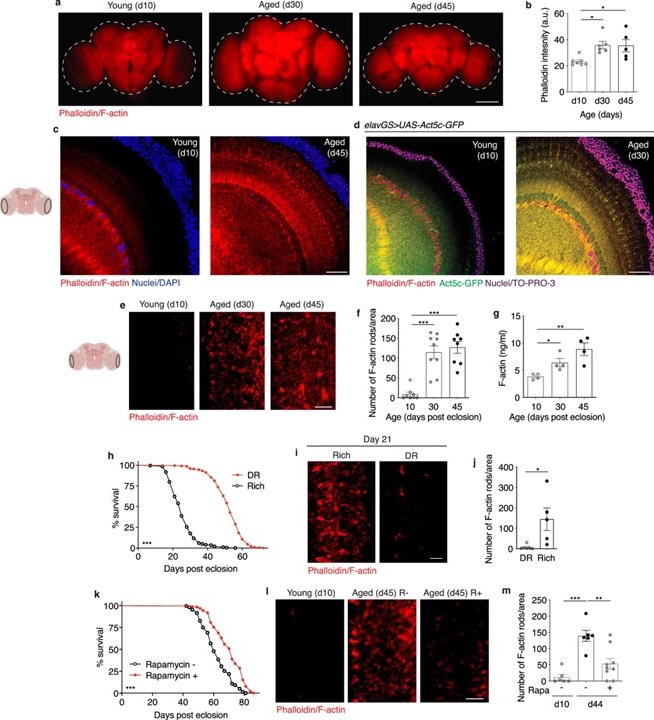
Actin is essential in cells, dictating shape, movement, and division. It exists in two forms: a monomeric form (G-actin) and a filamentous form (F-actin), which assembles into networks crucial for cell stability and function. Yet, as the UCLA team discovered, as Drosophila age, F-actin accumulates excessively in the brain. The buildup of these filaments disrupts cellular processes, which the team found to be a hallmark of brain aging and cognitive decline. When F-actin accumulates, the brain’s autophagy processes—its waste-clearing and recycling system—are impaired, leading to a chain reaction of cellular damage.
This disruption in autophagy, a vital mechanism for clearing damaged proteins and cellular debris, plays a pivotal role in many neurodegenerative diseases, including Alzheimer’s and Parkinson’s. Dr. Walker’s team revealed that F-actin accumulation in Drosophila impairs autophagy, leading to cognitive decline and reduced healthspan. However, by strategically targeting F-actin accumulation, they managed to reverse some aspects of brain aging in these flies, extending their healthspan and improving cognitive functions.
An Innovative Approach to Combat Brain Aging
The UCLA team employed various methods to explore how actin accumulation affects aging brains. They used genetic tools to inhibit F-actin in Drosophila neurons and observed remarkable changes: the reduction in F-actin buildup restored autophagic activity to youthful levels and alleviated aging-related cognitive decline. Their findings suggest that F-actin accumulation is not merely a consequence of aging but actively drives the aging process.
In aged Drosophila, the buildup of F-actin creates rod-like structures, or inclusions, in brain cells, which are absent in younger brains. Notably, these inclusions were not exclusive to the natural aging process. By using interventions known to prolong lifespan, like dietary restriction and rapamycin treatment, researchers observed a significant reduction in F-actin levels, even in older brains. This shows that these inclusions can be modulated, revealing an opportunity for targeted interventions.
Pharmacological and Genetic Interventions Yield Surprising Results
To further assess the impact of actin on aging, the team used two approaches: genetic inhibition of a protein called Fhos, which drives F-actin assembly, and pharmacological treatments with cytoskeletal drugs like cytochalasin D. In both cases, they observed improvements in cognitive performance and healthspan. This finding is significant because it implies that both genetic and pharmacological interventions could potentially delay brain aging by disrupting actin filament formation.
Phalloidin staining, a technique used to visualize actin, revealed that blocking Fhos in aging neurons prevented the formation of F-actin inclusions and restored memory and learning abilities in older flies. Incredibly, even brief treatments with cytochalasin D, a drug that disrupts actin polymerization, improved brain function in the aging flies, suggesting that reducing F-actin levels can reverse aging-related damage in brain cells.
The Potential of Actin-Targeted Interventions in Neurodegenerative Research
One of the study’s most compelling insights is that F-actin accumulation does not just impair cellular “clean-up” systems; it also affects the health of mitochondria, the cell’s powerhouse, which declines with age and is implicated in neurodegeneration. By reducing F-actin levels, the team also restored mitochondrial function, suggesting a link between actin dynamics and cellular energy maintenance. This discovery could shift the way scientists approach aging-related mitochondrial decline.
The study underscores the potential of targeting F-actin accumulation to restore cognitive function and improve healthspan in aging organisms. However, the team advises caution since while disrupting F-actin in neurons improved healthspan in Drosophila, applying this approach to other tissues may have different effects. Actin is critical in various cellular functions across the body, so future research will need to identify ways to selectively target actin in the brain.
Implications for Human Aging and Neurodegeneration
The implications of this research are vast, particularly for age-related diseases marked by cognitive decline. Conditions such as Alzheimer’s and Parkinson’s, known for abnormal intracellular inclusions that disrupt cellular function, may share pathological similarities with F-actin accumulation. The UCLA team’s work provides a foundation for exploring whether targeted actin modulation could offer therapeutic benefits in human neurodegenerative conditions.

Leave a Reply