In the rapidly advancing field of nanotechnology, the quest for precise and efficient drug delivery systems has led to the development of innovative solutions aimed at enhancing therapeutic outcomes while minimizing side effects. A notable breakthrough in this arena is the creation of artificial protein cages—nano-sized structures engineered to transport drugs directly to diseased cells.
The Innovation: Programmable Protein Cages
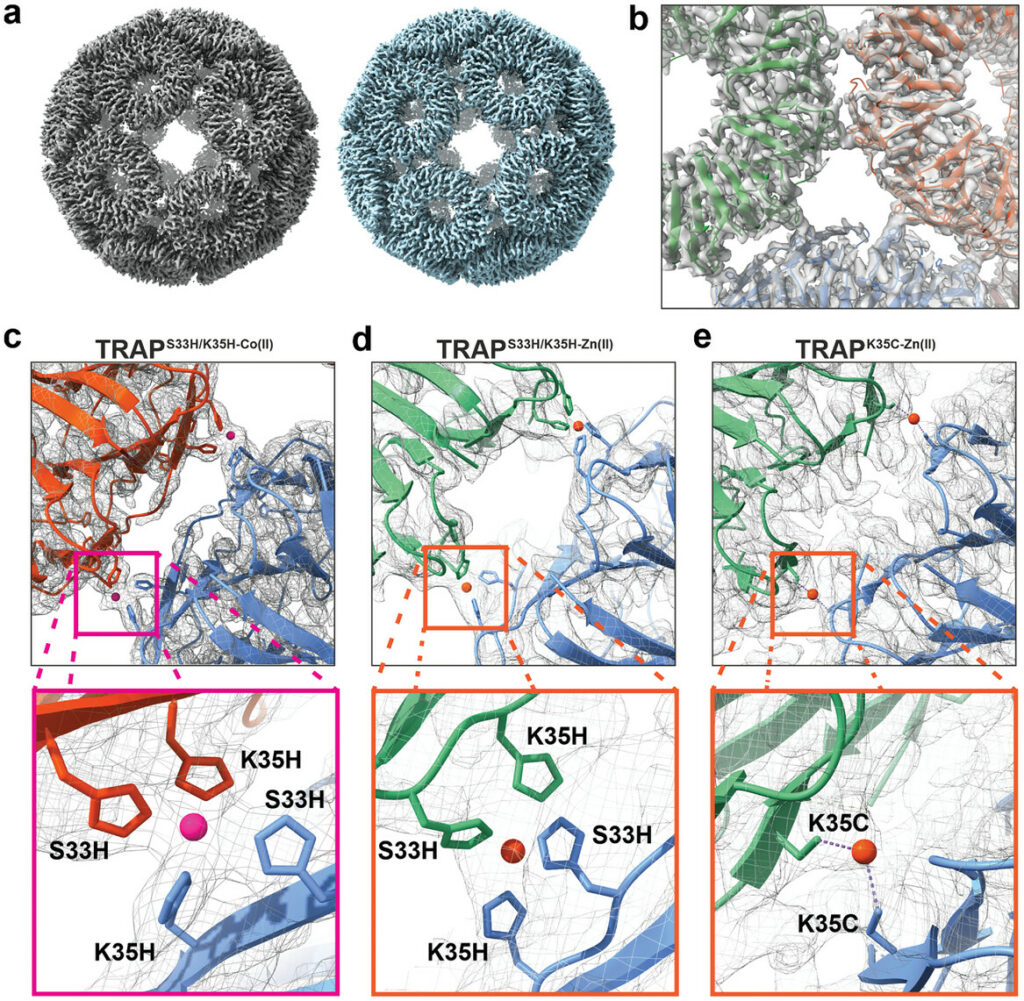
An international team of researchers from Durham University in the UK and the Malopolska Centre of Biotechnology at Jagiellonian University in Poland has engineered a novel protein cage. This artificial structure is constructed using a ring-shaped scaffold composed of TRAPs (trp RNA-binding attenuation protein). By introducing specific metal-binding sites, the researchers enabled these proteins to self-assemble into highly organized cages upon exposure to metal ions such as cobalt or zinc. These nano-scale structures feature a hollow core capable of encapsulating therapeutic agents.
What distinguishes this protein cage is its stability coupled with a programmable release mechanism. The cages remain intact under normal physiological conditions but can be triggered to disassemble and release their cargo in response to specific stimuli, such as changes in pH levels associated with certain diseases, including cancer. This pH-dependent assembly and disassembly render the protein cages highly adaptable for targeted drug delivery applications.
Dr. Jonathan Heddle of Durham University noted, “To have a highly stable nano-sized transport container that only opens up to release a toxic cargo when it reaches a diseased cell is a big challenge, and we think this work takes us a little closer to that goal.”
The Design Process: Engineering Precision at the Nanoscale
The creation of these protein cages involved a meticulous design process grounded in an understanding of protein-protein interactions and metal coordination chemistry. Leveraging the natural propensity of TRAP proteins to form ring-like structures, the researchers engineered metal-binding sites that dictate the assembly process. Upon introduction of specific metal ions, the TRAP rings self-assemble into larger, cage-like architectures. This method allows for precise control over the size and geometry of the cages, which is crucial for tailoring them to encapsulate various therapeutic molecules.
The programmability of the assembly process is a significant advancement. By selecting different metal ions or modifying the pH, the assembly and disassembly of the cages can be finely tuned. This tunability ensures that the protein cages remain stable during circulation in the bloodstream and release their payload only upon reaching the targeted diseased tissue, where environmental conditions differ from those in healthy tissues.
Implications for Drug Delivery: Targeted and Controlled Release
The development of such protein cages holds substantial promise for the field of drug delivery. Traditional drug delivery methods often suffer from a lack of specificity, leading to systemic side effects and reduced therapeutic efficacy. The ability of these protein cages to encapsulate drugs and release them in response to specific stimuli addresses these challenges by ensuring that the therapeutic agents are active only at the site of the disease.
Moreover, the biocompatibility and biodegradability of protein-based cages reduce the risk of adverse immune reactions, a common concern with synthetic nanocarriers. The use of naturally occurring amino acids in their construction means that, after fulfilling their drug delivery function, the cages can be safely metabolized by the body.
Future Directions: Bridging Research and Clinical Application
While the creation of these protein cages is a significant milestone, further research is necessary to transition from laboratory studies to clinical applications. Key areas for future investigation include assessing how these protein cages behave in living organisms in terms of circulation time, biodistribution, and ability to release drugs at the target site. Developing cost-effective and scalable methods for producing these protein cages in quantities sufficient for clinical use is also essential. Ensuring that the protein cages meet the safety and efficacy standards set by regulatory bodies for new drug delivery systems is another critical consideration.
The interdisciplinary nature of this research, combining principles from biochemistry, nanotechnology, and medicine, exemplifies the collaborative efforts required to advance drug delivery technologies.

Leave a Reply