A groundbreaking study carried out by the researchers of the University of California, San Francisco (UCSF) has unveiled a new way to combat cancer by reprogramming fat cells to outcompete tumors for essential nutrients. Researchers have developed a method called adipose manipulation transplantation (AMT), where engineered adipocytes—fat cells—are implanted near tumors to deprive them of the glucose and fatty acids they require for growth. By modifying these adipocytes to burn through energy at a higher rate, scientists found that tumors struggled to survive and, in many cases, shrank significantly.
The Science Behind Tumor Metabolism
Cancer cells have an insatiable appetite for nutrients, primarily glucose and fatty acids. In tumors, glucose uptake occurs at a significantly higher rate, leading to the production of lactate even when oxygen is available, and mitochondria are functioning properly. This phenomenon, widely researched, is referred to as the Warburg Effect. This effect allows them to rapidly metabolize glucose into energy and use it to fuel their uncontrolled growth. In oxygen-deprived environments, tumors further reprogram their metabolism to rely on fatty acids, which provide more energy per molecule than glucose.
Targeting cancer metabolism has long been a goal of oncologists, with several drugs attempting to block glucose and fatty acid uptake in tumor cells. However, directly suppressing these pathways in cancer patients can also affect healthy cells, leading to serious side effects. Instead, the researchers behind this new study proposed a different strategy: engineering fat cells that naturally compete with tumors for resources, effectively starving the cancer.
Engineering Energy-Hungry Adipocytes
The research team utilized CRISPR activation (CRISPRa) technology to modify white adipose tissue (WAT), which is commonly found in the human body, and transform it into brown adipose tissue (BAT)-like cells. Brown fat, unlike white fat, is highly metabolically active and burns large amounts of glucose and fatty acids to generate heat. By upregulating genes such as UCP1, PRDM16, and PPARGC1A, scientists induced this transformation in human adipocytes and observed a significant increase in their energy consumption.
In laboratory experiments, these engineered adipocytes were placed in close proximity to different types of cancer cells, including breast, pancreatic, and prostate cancers. The results were remarkable: cancer cell proliferation dramatically decreased as the tumors struggled to access enough nutrients to sustain their growth. The modified adipocytes were also able to lower glucose uptake and fatty acid oxidation in cancer cells, effectively cutting off their metabolic lifeline.
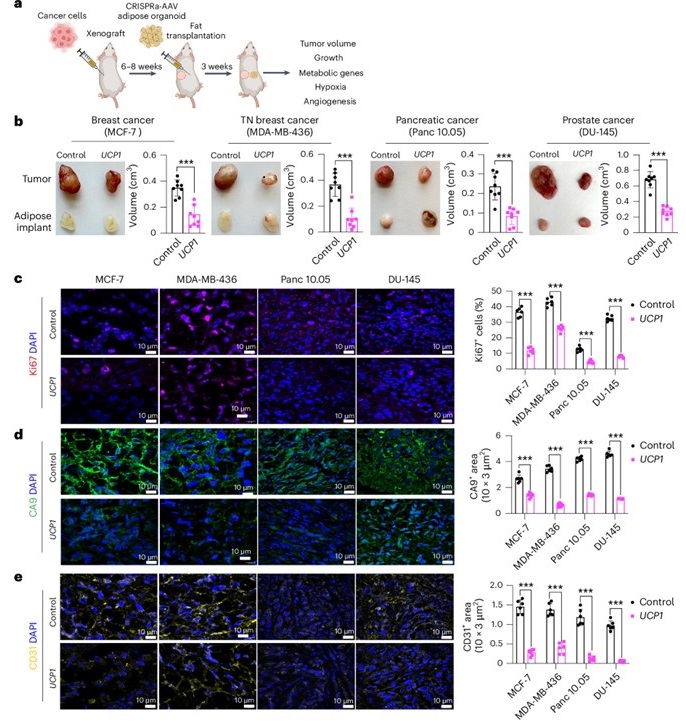
Success in Animal Models
To test the effectiveness of this approach in living organisms, researchers implanted these modified adipose organoids into genetically engineered mouse models of pancreatic and breast cancer. In both cases, tumor growth was significantly suppressed. The implanted fat cells not only outcompeted the tumors for glucose and fatty acids but also reduced angiogenesis—the formation of new blood vessels that supply tumors with nutrients. In addition, tumors showed less hypoxia, a condition of oxygen deprivation that typically fuels aggressive cancer progression.
Further studies on patient-derived breast cancer organoids revealed similar results. When human breast adipocytes were engineered with CRISPRa technology and co-cultured with cancer organoids from breast tumors, tumor proliferation significantly decreased. This suggests that AMT could be a promising therapeutic option for human cancer patients as well.
Personalized and Customizable Therapy
One of the most compelling aspects of this approach is its potential for customization. In addition to targeting glucose and fatty acid metabolism, researchers explored the ability of AMT to compete with tumors for other essential metabolites. For instance, they upregulated the UPP1 gene in adipose organoids, which allowed them to outcompete pancreatic ductal adenocarcinoma (PDA) cells for uridine, another key metabolic molecule used by some tumors. This further suppressed tumor growth, demonstrating that AMT could be adapted to different types of cancers based on their unique metabolic dependencies.
Moreover, the research team developed a controlled delivery system using biodegradable scaffolds to implant the engineered adipose organoids near tumors. This allows for easy removal or replacement of the therapy as needed. They also created an inducible version of the therapy using a tetracycline-controlled system, which enables gene activation to be turned on or off as required, providing a level of control and safety not seen in many existing cancer treatments.
A New Frontier in Cancer Therapy
This pioneering work introduces a potential novel strategy in cancer treatment—one that does not rely on toxic drugs or radiation but instead harnesses the body’s own fat cells to fight tumors. Unlike chemotherapy, which often comes with severe side effects, AMT presents a potentially safer and more targeted approach. Given that liposuction and fat transplantation are already common medical procedures, adapting this therapy for clinical use could be a relatively straightforward process.
While more research is needed before AMT can be widely applied in humans, the study’s results offer a promising glimpse into the future of cancer treatment. By reprogramming fat cells into energy-burning machines, scientists may have unlocked a new, effective way to combat cancer without harming healthy tissues. If successful in clinical trials, AMT could revolutionize how we approach cancer therapy, providing patients with a powerful new tool in their fight against this disease.




