MHRA Approves CASGEVY™ for Sickle Cell Disease and Transfusion-Dependent Beta Thalassemia, Marking a Milestone in Precision Medicine
Date: 7 December 2023
Introduction: In a historic move that signals a new era in medical innovation, Vertex Pharmaceuticals and CRISPR Therapeutics have received authorization from the United Kingdom’s Medicines and Healthcare products Regulatory Agency (MHRA) for the groundbreaking CRISPR/Cas9 gene-edited therapy, CASGEVY™ [exagamglogene autotemcel (Exa-cel)]. This cutting-edge therapy is set to transform the treatment landscape for individuals with Sickle Cell Disease (SCD) and Transfusion-Dependent Beta Thalassemia (TDT).
CASGEVY has been authorized for the treatment of eligible patients aged 12 years and older with SCD with recurrent vaso-occlusive crises (VOCs) or TDT without a human leukocyte antigen (HLA) matched hematopoietic stem cell donor. It is estimated that more than 2,000 patients in the UK are elegible for CASGEVY.
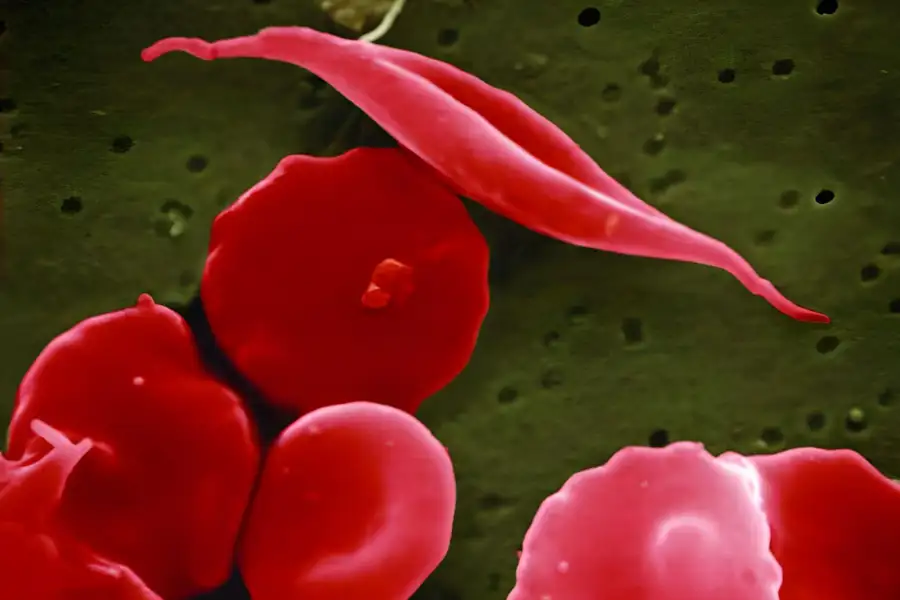
A Landmark Decision:
The approval from MHRA marks the first-ever authorization for a CRISPR/Cas9 gene-editing therapy. CASGEVY™ offers a ray of hope for patients suffering from these challenging hematological conditions, opening new possibilities for effective and precise treatments.
According to Reshma Kewalramani, M.D., Chief Executive Officer and President of Vertex, “Today is a historic day in science and medicine: this authorization of CASGEVY in Great Britain is the first regulatory authorization of a CRISPR-based therapy in the world.”
The Science Behind CASGEVY™:
CASGEVY™ is a genetically modified autologous CD34+ cell enriched population that contains human hematopoietic stem and progenitor cells, which in turn have been edited ex vivo by CRISPR/Cas9 in the erythroid-specific enhancer region of the BCL11A gene.
A high level of erythrocyte fetal hemoglobin (HbF) that comprises α- and γ-globins can improve symptomatology by mitigating sickle hemoglobin polymerization and erythrocyte sickling. BCL11A acts as a repressor of γ-globin expression and HbF production in adult erythrocytes: therefore, its down-regulation represented a possible therapeutic strategy for induction of HbF.
Benefits for Patients:
- Potentially Curative: CASGEVY™ is designed to be a one-time, potentially curative treatment. By addressing the genetic root causes of these diseases, it offers a transformative approach to patient care.
- Reduced Dependence on Transfusions: For individuals with Transfusion-Dependent Beta Thalassemia, CASGEVY™ has the potential to significantly reduce or eliminate the need for lifelong blood transfusions, improving both the quality of life and long-term health outcomes.
- Enhanced Quality of Life: Patients with Sickle Cell Disease often face chronic pain, organ damage, and a range of complications. CASGEVY™ holds the promise of alleviating these symptoms, providing patients with a chance at a healthier and more fulfilling life.
Global Implications:
The MHRA’s authorization of CASGEVY™ sets a precedent for other regulatory bodies worldwide. The therapy’s success could pave the way for broader acceptance of gene-editing technologies in the medical field, heralding a new era of precision medicine.
The future potentialities are clear for Samarth Kulkarni, Ph.D., Chairman and Chief Executive Officer of CRISPR Therapeutics: “I hope this represents the first of many applications of this Nobel Prize winning technology to benefit eligible patients with serious diseases.”
Challenges and Considerations:
While the authorization is a significant step forward, ongoing monitoring and research are crucial to understanding the long-term efficacy and potential side effects of CASGEVY™. As with any groundbreaking medical innovation, careful consideration of ethical, safety, and accessibility aspects remains paramount.
Looking Ahead:
The approval of CASGEVY™ represents a monumental achievement in the field of gene therapy, showing the potential of CRISPR/Cas9 technology to revolutionize medical treatments. Vertex Pharmaceuticals and CRISPR Therapeutics are now gearing up for the next phase — ensuring the therapy’s accessibility to those who stand to benefit the most.
The MHRA’s authorization of CASGEVY™ for the treatment of Sickle Cell Disease and Transfusion-Dependent Beta Thalassemia marks a pivotal moment in the history of medicine. This groundbreaking therapy stands as a testament to the power of precision medicine and the relentless pursuit of innovative solutions to address previously incurable genetic conditions. As CASGEVY™ takes its place on the frontlines of cutting-edge medical interventions, the hope is that it not only transforms the lives of individual patients but also paves the way for a future where gene-editing technologies become integral to the standard of care for a range of genetic disorders.

Leave a Reply