In a breakthrough study published in Nature Materials, scientists at the University of Stuttgart have unveiled an innovative approach to manipulating synthetic cells by leveraging reconfigurable DNA nanorobots. This research bridges the nanoscale and microscale worlds, offering a sophisticated method to reshape cell membranes and create functional synthetic channels, potentially revolutionizing synthetic biology.
The Role of Shape in Cellular Function
In biological systems, the shape of cells and their components plays a pivotal role in facilitating interactions and maintaining functionality. The plasma membrane, a vital cellular structure, combines lipids and proteins to form a flexible yet controlled barrier. While proteins regulate molecular transfer, both lipids and proteins contribute to the cell’s morphology, influencing its interactions with the environment. Synthetic biology aims to emulate these features by constructing functional cell models from the ground up.
However, achieving dynamic shape control in synthetic cells remains a challenge. Biological cells constantly reshape and respond to stimuli, while synthetic analogs often lack this adaptability. The new study tackles this limitation by introducing DNA-based tools capable of programmable, reversible, and morphological changes.
DNA Nanorafts: A Nanoscale Tool for Microscale Control
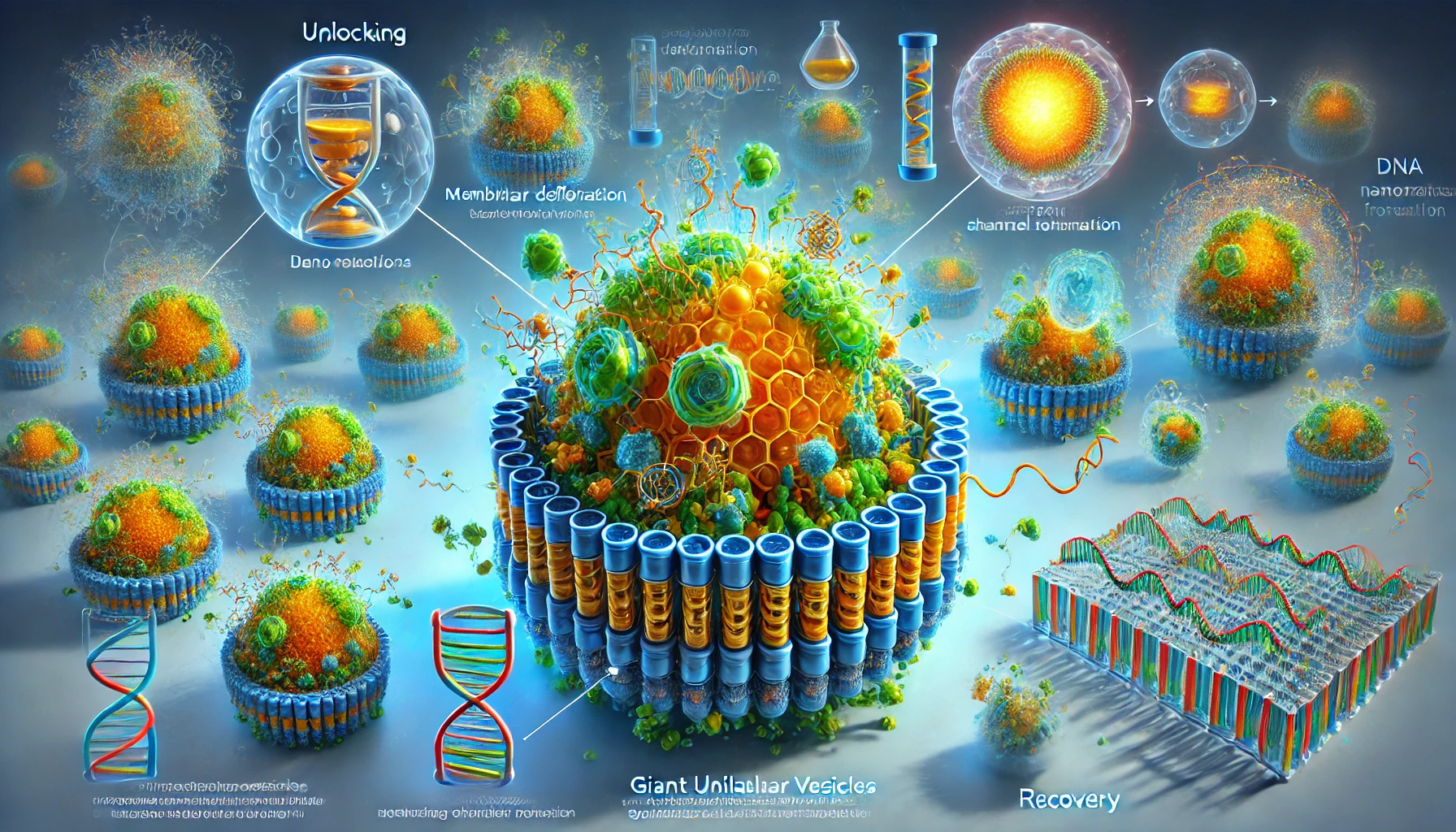
At the core of this innovation are DNA nanorafts, structures created using DNA origami techniques. These nanorafts are designed to transition between different shapes, such as squares and elongated rectangles, through specific DNA interactions. This transition is not merely cosmetic—it actively reshapes the synthetic cell membranes to which they are attached.
The system relies on giant unilamellar vesicles (GUVs) as synthetic cell models. When DNA nanorafts bind to the vesicle membrane, their structural changes create steric pressure, leading to deformations of the GUV surface. The result is a striking interplay where nanoscale changes in DNA configuration directly translate to microscale transformations in the synthetic cell’s morphology.
Crucially, these morphological changes are reversible. By introducing specific DNA sequences, the nanorafts can return to their original shape, allowing the GUVs to recover their spherical form. This reversible functionality adds a layer of precision and control previously unattainable in synthetic cell engineering.

Synthetic Channels: A New Frontier in Membrane Functionality
One of the most significant outcomes of this study is the creation of synthetic channels within the GUV membranes. As the DNA nanorafts self-arrange into ordered domains, they perforate the membrane, forming functional channels. These channels enable the transport of molecules, including larger entities like green fluorescent protein (GFP), across the membrane.
The ability to regulate membrane permeability with such precision is a notable advancement. Synthetic channels formed by DNA nanorafts are unique in that they can be sealed and reopened on demand. This feature allows for controlled transport of cargo, a capability that surpasses the limitations of natural protein-based pores.
Exploring the Dynamics of DNA Nanorafts and GUVs
The researchers meticulously examined the parameters influencing DNA raft and GUV interactions. Factors such as DNA raft density, cholesterol-anchor configuration, and osmotic pressure all played a role in determining the extent of GUV deformation and channel formation. At optimal conditions, the DNA nanorafts induced significant changes, highlighting the importance of fine-tuning these variables for maximum efficiency.
The channels themselves exhibited a size range capable of transporting molecules up to 70 kilodaltons, making them versatile for various applications. The system also demonstrated stability and adaptability, ensuring that the channels remained functional over extended periods.
Applications and Implications
The ability to reshape synthetic cells and regulate their functionality opens up numerous possibilities in biotechnology and medicine. Controlled cargo transport through synthetic channels can be applied in drug delivery systems, where therapeutic agents are released directly into targeted areas. Similarly, the dynamic reshaping of membranes could be utilized in biosensing platforms, allowing for real-time adaptation to environmental changes.
Beyond biomedical applications, this technology has implications for fundamental research. By recreating and surpassing natural cellular processes, scientists can gain deeper insights into the mechanics of life while designing entirely new functionalities. The modularity of DNA nanorafts further allows for customization, enabling researchers to tailor synthetic cells for specific tasks.
Challenges and Future Directions
Despite its promise, this technology is not without challenges. The stability of DNA nanostructures in complex biological environments and their scalability for large-scale applications remain areas of ongoing research. Additionally, integrating these systems with existing biotechnological platforms requires further development.
Nevertheless, this study represents a significant step forward. By combining the programmability of DNA nanotechnology with the versatility of synthetic biology, this research lays the groundwork for future advancements. The potential to design dynamic, functional, and synthetic cells that can interact with their environment in unprecedented ways underscores the transformative impact of this work.