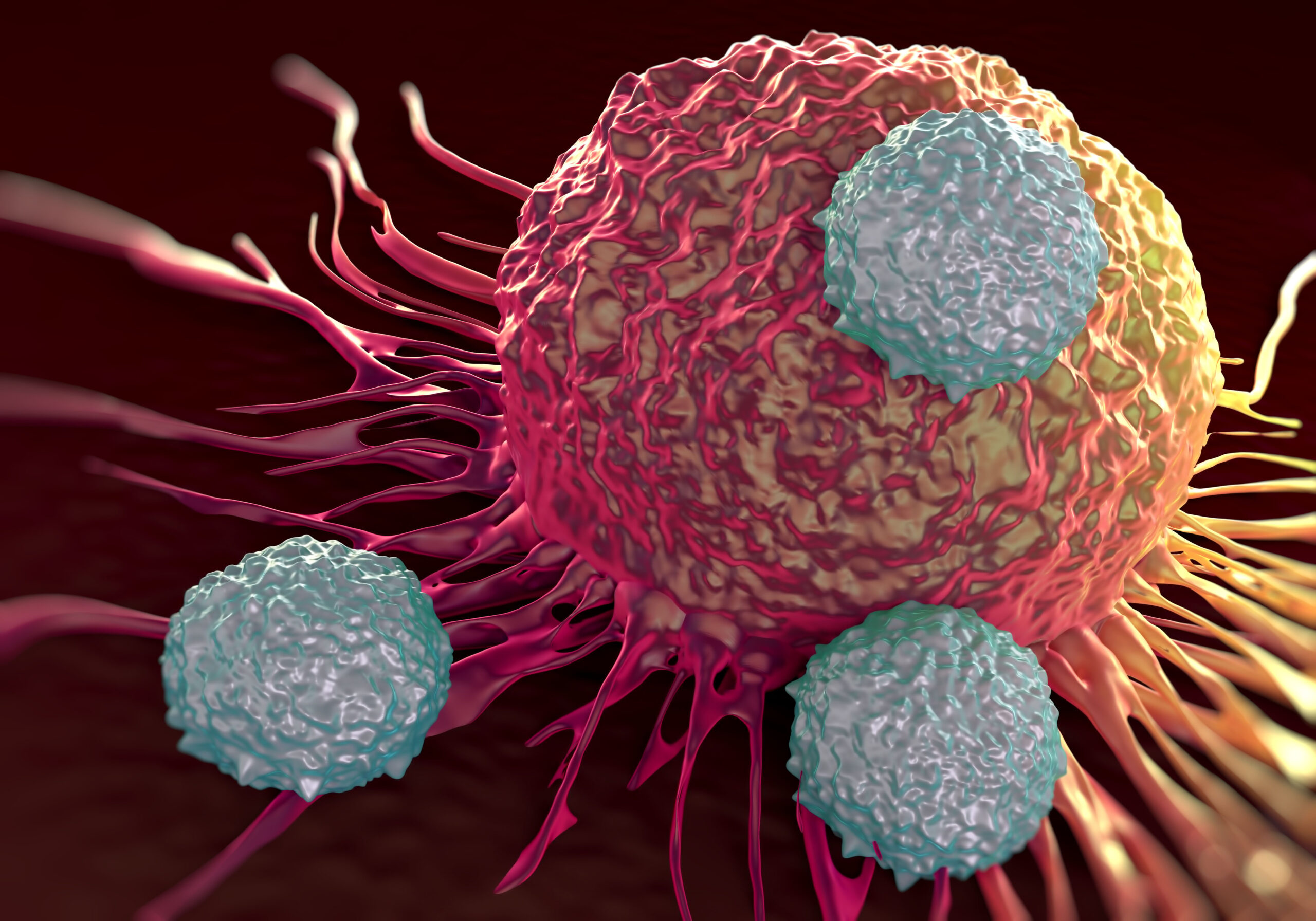
Harnessing Nature’s Superpower: Scientists Unleash Engineered T-Cells to Combat Solid Tumors
In a groundbreaking paper titled "Naturally occurring T cell mutations enhance engineered T-cell therapies," scientists from the University of California, San Francisco (UCSF) and Northwestern Medicine have unveiled a revolutionary…