In a groundbreaking study recently published in Nature Aging, researchers led by a team at Cold Spring Harbor Laboratory (CSHL) have unveiled a revolutionary approach to address age-related metabolic dysfunction. The paper, titled “Prophylactic and long-lasting efficacy of senolytic CAR T cells against age-related metabolic dysfunction,” introduces an innovative anti-aging therapy centered around chimeric antigen receptor (CAR) T cells. These specialized immune cells target senescent cells, which accumulate in organisms as they age and are believed to be the culprits behind numerous age-related diseases.
Cellular Senescence
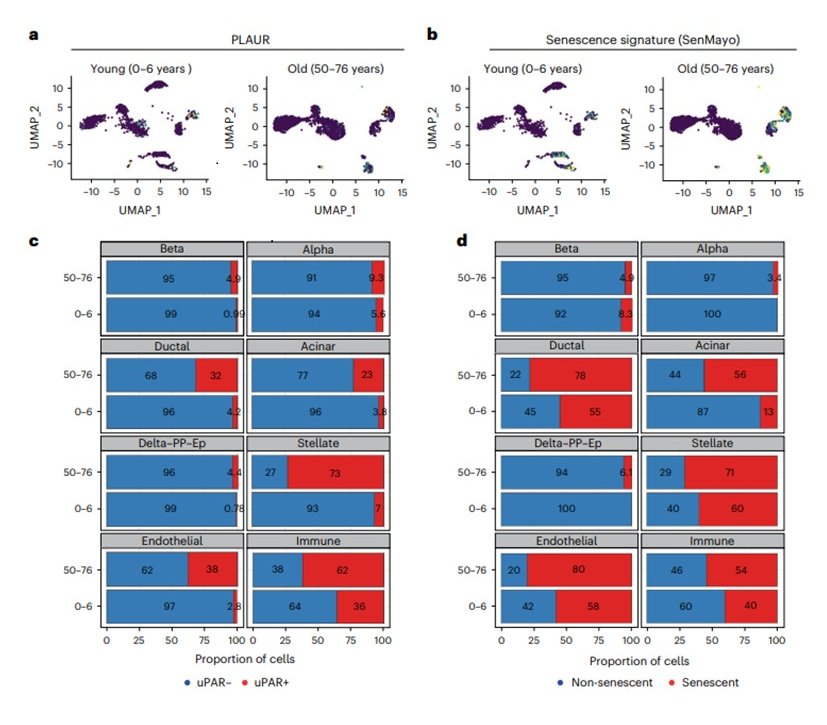
The head of the research team, Assistant Professor Corina Amor Vegas, MD, PhD, explained the significance of their findings, stating, “Cellular senescence is a stress response program characterized by stable cell cycle arrest and the production of the senescence-associated secretory phenotype (SASP), which includes pro-inflammatory cytokines and matrix remodeling enzymes.” This insight into the nature of senescent cells paved the way for the development of a targeted therapeutic strategy.
Senolytic approach
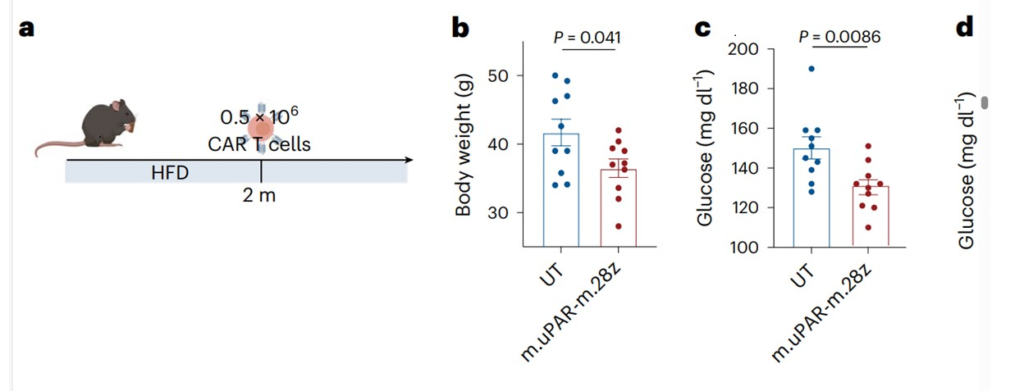
The study focused on senolytic approaches based on uPAR CAR T cells that target the cell surface protein uPAR which is overly expressed in senolytic cells. Compared to the control, animals treated with uPAR CAR T cells showed lower body weight, increased physical activity, and improved metabolism and glucose tolerance, without presenting tissue damage or toxicity. These results highlight the capacity of uPAR CAR T cells to remove senescent uPAR-positive cells safely and effectively.
The approach is long-lasting and effective, as Dr. Vegas and her team found that a single low dose of uPAR CAR T cells caused a significant impairment in age-induced or high-fat diet (HFD)-induced metabolic syndrome. Importantly, these effects persisted, highlighting the promise of this innovative therapy in combating age-related health issues.
Single-dose administration
Unlike conventional small-molecule approaches, CAR T cells offer a distinct advantage. Dr. Vegas highlighted this, stating, “Unlike small molecules, CAR T cells only require that the target antigen is differentially expressed on target cells compared to normal tissues; moreover, as ‘living drugs,’ these therapeutics have the potential to persist and mediate their potent effects for years after a single administration.” All this is based on the ability of T cells to develop a memory and persist in the body for a long time, unlike chemical drugs whose effect is limited to a brief interval after administration.
This longevity in effect could revolutionize the landscape of anti-aging interventions.
Further Confirmation from High-Fat Diet
The study also involved treating mice during their youth and administering therapy in response to a high-fat diet, mimicking conditions that often lead to metabolic dysfunction in humans. Also in this case, when compared to the control, the treated animals showed lower body weight and improved glucose and insulin tolerance.
Improved healthspan
The findings from this research have significant implications for the field of anti-aging therapeutics. With a focus on cellular senescence, this innovative approach could pave the way for interventions that not only extend lifespan but also enhance healthspan—the period of life spent in good health and free from serious illness.
As the research community grapples with the complexities of aging, the work led by Dr. Vegas and her team offers a ray of hope. The unique characteristics of CAR T cells, coupled with their ability to target senescent cells, open new avenues for developing interventions that go beyond merely extending life to actively improving the quality of life in later years.




