In a recent publication in Cell Discovery titled “Engineered, nucleocytoplasmic shuttling Cas13d enables highly efficient cytosolic RNA targeting,” researchers from Helmholtz Munich and the Technical University of Munich (TUM) unveiled a groundbreaking advancement in the realm of genetic engineering. Their work introduces a novel tool, Cas13d-NCS, designed to revolutionize the efficacy of CRISPR RNA molecules within the cell, particularly in combating RNA viruses. This development is a response to the inefficiency of existing RNA-targeting tools like CRISPR/Cas13 in the cytoplasm, where many RNA viruses thrive. By enabling precise targeting and neutralization of cytosolic RNA, Cas13d-NCS promises to usher in a new era of precision medicine and advanced viral defense strategies.
Enhancing CRISPR’s Reach: The Need for Cas13d-NCS
The CRISPR/Cas13 systems have garnered attention for their programmable nature, allowing for precise manipulation of RNAs across various applications. Among the Cas13 subtypes, Cas13d stands out for its heightened activity in mammalian cells, making it a promising candidate for antiviral interventions in human. However, its effectiveness has been hindered by its limited activity in the cytosol, where most RNA viruses replicate. This limitation has hampered the development of programmable antivirals, as current methods imply an uncontrolled nuclear leakage of CRISPR components.
Engineering Precision: Cas13d-NCS Unveiled
To address this challenge, the researchers engineered Cas13d-NCS, a nucleocytoplasmic shuttling system capable of transporting CRISPR RNA molecules from the nucleus to the cytosol. This breakthrough overcomes the fundamental barrier of Cas13d’s nuclear localization, thereby expanding its utility in targeting cytosolic RNA. Through meticulous screening and optimization, the team developed a solution that outperforms its predecessors in degrading mRNA targets and neutralizing self-replicating RNA viruses.
As the investigators explain: “CRISPR/Cas13 systems are programmable tools for manipulating RNAs and are used in a variety of RNA-targeting applications. Within the Cas13 family, Cas13d is the most active subtype in mammalian cells. Recently, Cas13d was harnessed as an antiviral against diverse human RNA viruses.”
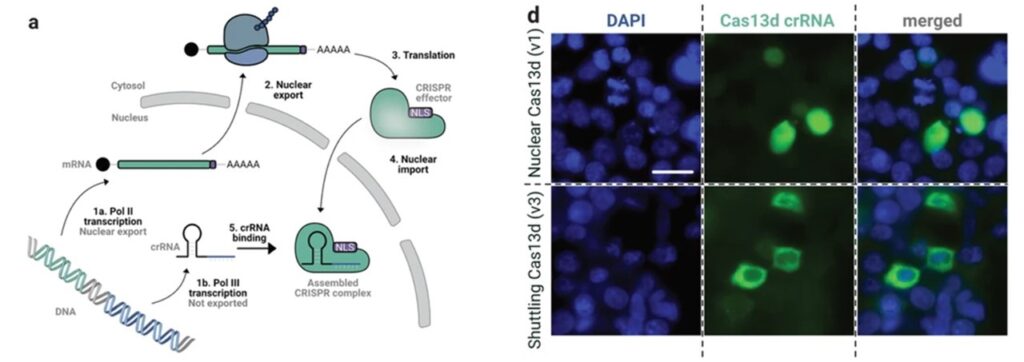
Unleashing the Potential: Applications of Cas13d-NCS
Cas13d-NCS demonstrates unparalleled efficacy in targeting a spectrum of RNA viruses, including variants of SARS-CoV-2 and the Venezuelan equine encephalitis virus. By precisely guiding the CRISPR-Cas complex to specific RNA sequences in the cytosol, this tool offers a powerful strategy for combating viral infections. Its versatility and precision hold promise for a wide range of applications beyond traditional antiviral strategies, paving the way for personalized medicine.
Wolfgang Wurst, PhD, director of the Institute of Developmental Genetics at Helmholtz Munich and coordinator of the study, lauds the significance of this step forward: “This breakthrough in antiviral development with Cas13d-NCS marks a pivotal moment in our ongoing battle against RNA viruses,” says Wurst, who continues: “this achievement showcases the power of collaborative innovation and human ingenuity.”
Looking Ahead: Future Implications
As the scientific community continues to explore the potential of CRISPR technologies, the development of Cas13d-NCS represents a new hope in genetic engineering. Its ability to precisely manipulate subcellular localization opens avenues for targeted interventions and therapeutic innovations. With further research and refinement, Cas13d-NCS holds promise for addressing diverse challenges in biomedicine, from viral infections to genetic disorders, heralding a future defined by precision medicine and advanced antiviral strategies.