A groundbreaking study published in Bone Research reveals a novel approach to creating high-quality mitochondria that could revolutionize treatments for osteoarthritis and other metabolic disorders. Researchers from Zhejiang University in China have developed an “organelle-tuning condition” that dramatically increases both the quantity and quality of mitochondria produced from human mesenchymal stem cells.
The Mitochondrial Challenge in Medicine
Mitochondria, often described as the powerhouses of cells, provide approximately 90% of the energy required for human life. When these critical organelles malfunction, various diseases can develop, ranging from neurodegenerative conditions to heart disease and metabolic disorders.
Mitochondrial transplantation has emerged as a promising therapeutic approach, with research showing benefits for conditions like ischemic heart disease, mitochondrial DNA disorders, and wound healing. However, the widespread application of this technique faces a significant obstacle: the limited availability of healthy mitochondria.
Current methods rely on harvesting mitochondria from liver or muscle tissue, which cannot sustainably meet clinical demand. A single treatment for ischemia-reperfusion injury can require over 10^9 mitochondria per patient, presenting a substantial challenge for therapeutic applications.
A New Approach: The Mitochondria Factory
The researchers tackled this challenge by developing what they call a “mitochondria-producing strategy” that manipulates mitobiogenesis (the creation of new mitochondria) and tunes organelle balance within human mesenchymal stem cells (MSCs).
Through a systematic screening process, they identified a specialized culture medium containing nine key components, including basic fibroblast growth factor, vitamin C, hydrocortisone, and human platelet lysate. When MSCs were cultured in this “mito-condition,” they exhibited remarkable changes in both quantity and quality of mitochondria.
The results were striking: an 854-fold increase in mitochondrial production compared to standard cell culture methods was achieved. Furthermore, these mitochondria weren’t just more numerous—they demonstrated significantly enhanced function, with ATP production levels 5.71 times higher than mitochondria from cells grown under typical conditions. This remarkable improvement was sustainable through continuous cell passage, enabling long-term production.
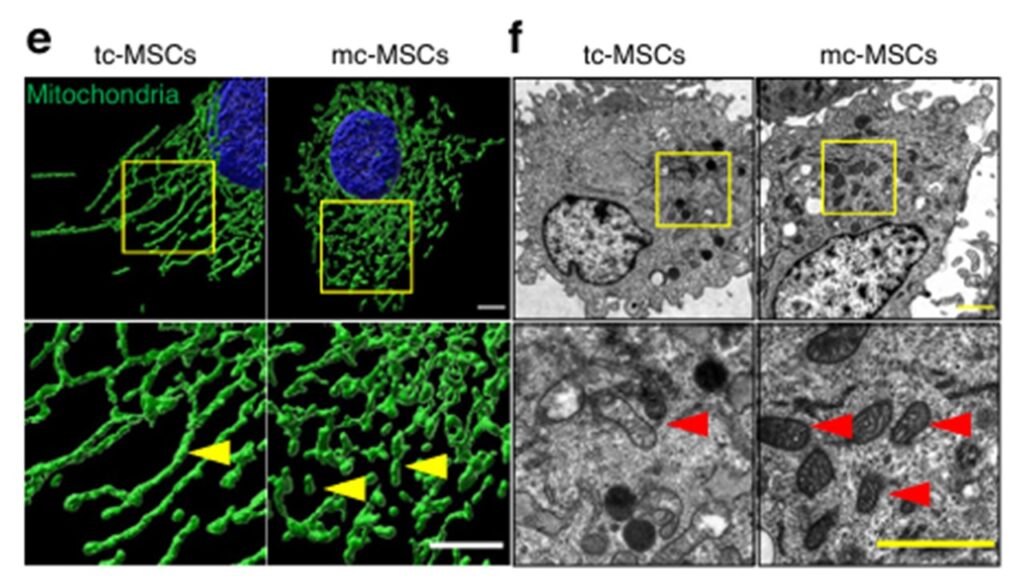
The Science Behind Enhanced Production
What makes this approach particularly innovative is how it reprograms cellular metabolism. Using transcriptomic analysis, the team discovered that the mito-condition activates the AMPK (AMP-activated protein kinase) pathway, which serves as an energy sensor in cells.
This activation establishes what the researchers describe as a novel cellular state ideal for mitochondrial fabrication. In this state, cells demonstrate enhanced proliferation, with population doubling time decreasing from 38.45 to 21.23 hours. The cells also show accelerated mitobiogenesis through increased expression of mitochondrial biogenesis-related genes.
Perhaps most interestingly, the researchers observed suppression of energy-consuming activities like autophagy, cell migration, and secretion. This suggests the mito-condition creates a specialized cellular state where resources are directed toward rapid cell cycling and mitochondrial synthesis instead of being used for other cellular processes.
This artificially tuned state provides insights into organelle homeostasis and could induce the development of new approaches in organelle therapy beyond mitochondria.
Proving Effectiveness in Osteoarthritis Treatment
To demonstrate the therapeutic potential of these high-quality mitochondria, the team tested them in a mouse model of osteoarthritis (OA), a common joint disease characterized by cartilage degeneration and associated with mitochondrial dysfunction in chondrocytes (cartilage cells).
The results were compelling. Mice treated with mitochondria produced under the mito-condition showed superior outcomes in cartilage repair compared to those treated with conventionally produced mitochondria. There was better preservation of cartilage functional markers, including collagen type II and aggrecan. The treatment also reduced osteophyte formation and subchondral bone sclerosis, key indicators of OA progression.
Efficiency was another significant advantage. For an equal yield of mitochondria, the mito-condition approach saved 44.66% of expansion time or 66.72% of initial cells compared to typical methods. This efficiency could dramatically reduce costs and time required for mitochondrial production in clinical settings.
Implications for Future Treatments
This research represents a significant advancement in the field of regenerative medicine, offering a sustainable way to produce high-quality human mitochondria for clinical use. The approach could potentially provide sufficient mitochondria for a substantial number of patients requiring mitotherapy.
The strategy enables the production of approximately 10^13 off-the-shelf mitochondria within 15 days, which could meet the growing demand for mitochondrial treatments. This industrial-scale production capability addresses one of the major limitations currently hampering the widespread clinical application of mitochondrial therapy.
Beyond osteoarthritis, this technique could benefit patients with various conditions involving mitochondrial dysfunction, including ischemic heart disease, mitochondrial DNA disorders, infertility, wound healing, and other metabolic syndromes. The versatility of this approach makes it particularly valuable as researchers continue to explore the therapeutic potential of mitochondrial transplantation.
Challenges and Future Directions
While the results are promising, certain limitations remain in the current study. There is limited evidence for efficient mitochondria transfer and retention in targeted cells after transplantation. Future research will need to focus on developing advanced mitochondria delivery systems and validating mitochondria functionality after they have been transplanted into host tissues.
Additionally, further investigation is needed to fully understand the molecular mechanisms underlying the enhanced mitochondrial production and function observed in the study. A deeper understanding of these processes could lead to even more efficient production methods and potentially new therapeutic targets.
Paolo Rega

Leave a Reply