By Paolo Rega
In the high-stakes battle against tuberculosis and other mycobacterial infections, scientists are turning to an unlikely ally: viruses that infect bacteria, known as bacteriophages. A groundbreaking study published in Cell by Krista Freeman and colleagues unveils the atomic-level structure and infection dynamics of one such virus—mycobacteriophage Bxb1—offering new hope for precision phage therapy and next-generation vaccine platforms.
The research combines cutting-edge cryo-electron microscopy (cryo-EM) and cryo-electron tomography (cryo-ET) to provide an unprecedented look at how Bxb1 latches onto and infiltrates Mycobacterium smegmatis, a stand-in for more dangerous cousins like Mycobacterium tuberculosis. The insights are not only a tour de force in molecular imaging but may also chart a course toward therapeutic applications for drug-resistant infections.
A Virus with a Purpose
Bxb1 isn’t just any bacteriophage. It’s a well-characterized member of the actinobacteriophage family with a track record in genetic engineering and potential in biomedical innovation. Researchers have long admired its ability to integrate into bacterial genomes—a feature already harnessed in synthetic biology. But until now, the intricate machinery Bxb1 uses to infect its host remained a mystery.
Engineering Meets Biology: A Structural Symphony
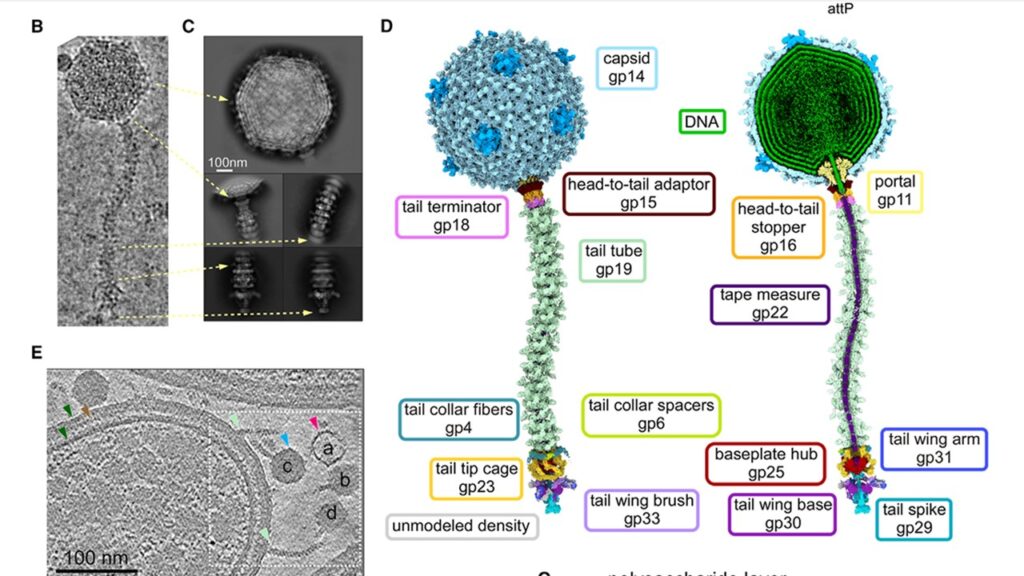
The team started by using cryo-EM to freeze Bxb1 particles in action, capturing them in exquisite detail. At the heart of the phage is its 60-nanometer-wide capsid, an icosahedral protein shell packed with genetic material. This shell connects to a long, flexible tail—about 130 nanometers in length—that acts as a delivery system, guiding the viral DNA into the host.
What makes Bxb1 truly stand out, however, is its elaborate architecture. The virus is covered in protein domains known as C-terminal extensions (CTEs), decorating both its capsid and tail tube. These CTEs not only stabilize the structure but are also highly immunogenic, making them promising candidates for displaying vaccine antigens.
More intriguingly, Bxb1 uses a mix of symmetrical protein complexes—3-, 5-, 6-, and 12-fold structures—that fit together like intricate puzzle pieces. This allows it to manage complex transitions between different parts of its body, such as the “portal” that connects the capsid to the tail. Think of it as a molecular gearbox that shifts the virus from packaging to delivery mode.
Tail Tip Tango: Piercing the Host
One of the most elusive—and critical—moments in a phage’s life is when it infects a host. The research team used cryo-ET, a technique that captures 3D images of host-bound phages frozen in the act, to observe this step in real-time. They focused on how Bxb1 tail tips interact with the bacterial surface and undergo dramatic rearrangements.
The tail tip, it turns out, is no static needle. It is a dynamic assembly of nine proteins, including a specialized “cage” that unfurls like a flower to interface with the host’s thick, waxy cell wall. This structure flattens into a disc-like shape as the phage binds, potentially prying open the outer membrane.
The team also discovered signs of enzymatic action. One tail component, called gp30, appears to contain a D-alanyl-D-alanine carboxypeptidase domain—a tool thought to help digest the bacterial cell wall and clear a path for viral DNA to enter.
Watching the Infection Unfold
With cryo-ET snapshots at different stages, the researchers could distinguish between virions still packed with DNA and those that had already ejected it. Remarkably, the tail tube acts as a pipeline, and subtle changes—like the disappearance of a protein known as the tape measure protein (TMP)—signal the handoff of genetic material into the host.
This direct observation of Bxb1’s DNA delivery is a milestone. It shows that genome ejection does not require a massive restructuring of the phage’s body. Rather, it is more like opening a valve in an already-built conduit.
A Trojan Horse for Vaccines?
Beyond its infection mechanics, Bxb1 is being eyed as a vaccine delivery vehicle. Its CTEs could serve as platforms to present antigens—foreign proteins that trigger immune responses.
However, the study uncovered constraints: while CTEs are removable, they cannot be easily replaced with bulky antigens without destabilizing the structure. The researchers propose a workaround—using flexible linkers or co-expressing stabilizing proteins to hold the viral scaffold together.
The Bigger Picture: Toward Phage Therapy
Bxb1 is just one of over 2,600 sequenced mycobacteriophages, but it is the first to be characterized so completely at an atomic level. Understanding how phages like Bxb1 infect bacteria could pave the way for targeted phage cocktails to treat drug-resistant infections, especially in cases where antibiotics fail.
That’s not science fiction. Compassionate phage therapy has already saved lives in a handful of tuberculosis and Mycobacterium abscessus cases. Yet tailoring phages to specific bacterial strains remains a hurdle—one that structural insights like these could help overcome.


Leave a Reply